- Home
- Veterinary
Brains Bioceutical is Shaping the future of CBD in Veterinary Medicine
An evidence-based and science-led pioneer of cannabinoid health & wellness solutions
Navigating New Frontiers in Veterinary Health. CBD Pet Products. Made Better.
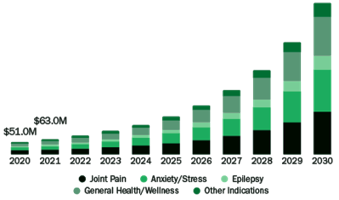
CBD is predicted to be a huge disruptor in the Pet Industry by 2025. Enhanced Understanding and Innovation: Unveiling the Benefits of CBD for Pets. Brains Bioceutical is the leader in evidence-based Phyto-cannabinoid health solutions to enhance life and treatment options for all.
Brains Bio Cannabinoid Innovation Platform
Brains Bioceutical offer a unique innovation platform to unlock tailored cannabinoid-based pharmaceuticals to expand treatment options and convenience for canine, feline, equine and more worldwide.
High quality APIs
- Robust cannabinoid API portfolio with rich pipeline of minor cannabinoids
Customized solutions
- Formulated APIs and Drug product R&D
- Customized, bioavailability enhanced finished dosage forms for clinical trials, plus formulation, technical & application technology support
Expert services
- Quality, regulatory and scientific expertise in early-stage drug development and beyond
- Access to clinical data packages and co-development opportunities
- Cannabinoid portfolio development pathway

Purpose
Improving Quality of Life for animals through evidence-based and science-led cannabinoid health & wellness solutions.
Our dedication to innovation, quality, and comprehensive care sets us apart, as evidenced by our specialized offerings in nutraceuticals, supplement products, and pharmaceuticals.
- Real world evidence for CBD use in animals is compelling
- CBD is forecast to be a significant disrupter in the animal health market
- Growing bank of early-stage CBD clinical research around the world
- Opportunity for first mover advantage in many markets
Quality
Brains proudly stands at the forefront of developing advanced veterinary solutions, with a strong commitment to quality and safety. Our state-of-the-art manufacturing facility adheres to the stringent standards of EU-GMP compliance and boasts registration with the UK’s Medicine and Healthcare products Regulatory Agency (MHRA). This accreditation underscores our capability to produce and distribute top-grade phytochemical APIs for both human and veterinary medicinal products.


Production
Located in Kent, England, our operations are housed within two facilities that meet the rigorous standards of EU-GMP compliance. These sites have been thoroughly inspected and are registered with the UK’s Medicines and Healthcare products Regulatory Agency (MHRA), reflecting our adherence to the highest standards of quality and safety in production.
Our facilities boast an expansive 25,000 square feet of operational space, designed to facilitate efficient and large-scale production. With a robust CBD production capacity of 12.6 tons per annum, we are well-equipped to meet the growing demand for phyto-cannabinoid products, ensuring that our partners and customers have consistent access to high-quality solutions.
Strategic Partnering Opportunities with Key Players
Unlock customized cannabinoid-based pharmaceuticals to expand treatment possibilities
Drug Substance, Intermediates & Products
Supply of Customized Solutions
• Highly pure, standardised EU GMP Cannabinoid APIs
• IP protected drug intermediates (CBD oral solid dosage forms)
• Finished drug formats
End-to-End Services
DP R&D Services and 3rd Party CDMOs
• Quality, regulatory and scientific expertise (incl. Clinical trial design)
• Drug delivery technologies and supporting data packs
Access to Clinical Data
Utilization of proprietary clinical trial data
• Access to indication specific data
• Access to data for clinical program design and execution
• Rights to clinical data packages
Commercial Collaboration Options
• Exclusive supply of product for specific indications or markets
• Co-development of API or enhanced API formulations
• Co-development of clinical programs furthering existing Concepts
• License or option to clinical data packs and approved cannabinoid drugs
• Early market access licensing of existing drug products
• End-to end services agreement

A Unique Suite of Licenses Equipped to Lead the Market
• MHRA registration
• GMP Certification for Human & Veterinary Medicines
• GDP Certification
• Schedule 1 Controlled Substances License
• THC-free, 100% natural hemp derived
• Highest purity, 100% CBD (98.0 –102.0%)
• Consistent batch quality
• Ethanol, preservative, flavoring-free
• ICH-Q7 compliant 3 year reset date stability

